Deposition is a major problem in the operation of steam generating equipment. The accumulation of material on boiler surfaces can cause overheating and/or corrosion. Both of these conditions frequently result in unscheduled downtime.
Boiler feedwater pretreatment systems have advanced to such an extent that it is now possible to provide boilers with ultrapure water. However, this degree of purification requires the use of elaborate pretreatment systems. The capital expenditures for such pretreatment equipment trains can be considerable and are often not justified when balanced against the capability of internal treatment.
The need to provide boilers with high-quality feedwater is a natural result of the advances made in boiler performance. The ratio of heating surface to evaporation has decreased. Consequently, heat transfer rates through radiant water wall tubes have increased-occasionally in excess of 200,000 Btu/ft²/hr. The tolerance for deposition is very low in these systems.
The quality of feedwater required is dependent on boiler operating pressure, design, heat transfer rates, and steam use. Most boiler systems have sodium zeolite softened or demineralized makeup water. Feedwater hardness usually ranges from 0.01 to 2.0 ppm, but even water of this purity does not provide deposit-free operation. Therefore, good internal boiler water treatment programs are necessary.
Common feedwater contaminants that can form boiler deposits include calcium, magnesium, iron, copper, aluminum, silica, and (to a lesser extent) silt and oil. Most deposits can be classified as one of two types (Figure 12-1):
- scale that crystallized directly onto tube surfaces
- sludge deposits that precipitated elsewhere and were transported to the metal surface by the flowing water
Scale is formed by salts that have limited solubility but are not totally insoluble in boiler water. These salts reach the deposit site in a soluble form and precipitate when concentrated by evaporation. The precipitates formed usually have a fairly homogeneous composition and crystal structure.
High heat transfer rates cause high evaporation rates, which concentrate the remaining water in the area of evaporation. A number of different scale-forming compounds can precipitate from the concentrated water. The nature of the scale formed depends on the chemical composition of the concentrated water. Normal deposit constituents are calcium, magnesium, silica, aluminum, iron, and (in some cases) sodium.
The exact combinations in which they exist vary from boiler to boiler, and from location to location within a boiler (Table 12-1). Scale may form as calcium silicate in one boiler and as sodium iron silicate in another.
Compared to some other precipitation reactions, such as the formation of calcium phosphate, the crystallization of scale is a slow process. As a result, the crystals formed are well defined, and a hard, dense, and highly insulating material is formed on the tube metal. Some forms of scale are so tenacious that they resist any type of removal-mechanical or chemical.
Sludge is the accumulation of solids that precipitate in the bulk boiler water or enter the boiler as suspended solids. Sludge deposits can be hard, dense, and tenacious. When exposed to high heat levels (e.g., when a boiler is drained hot), sludge deposits are often baked in place. Sludge deposits hardened in this way can be as troublesome as scale.
Once deposition starts, particles present in the circulating water can become bound to the deposit. Intraparticle binding does not need to occur between every particle in a deposit mass. Some nonbound particles can be captured in a network of bound particles.
Table 12-1. Crystalline scale constituents identified by X-ray diffraction.
| Name | Formula |
| Acmite | Na2OFe2O34SiO2 |
| Analcite | Na2OAl2O34SiO22H2O |
| Anhydrite | CaSO4 |
| Aragonite | CaCO3 |
| Brucite | Mg(OH)2 |
| Calcite | CaCO3 |
| Cancrinite | 4Na2OCaO4Al2O32CO29SiO23H2O |
| Hematite | Fe2O3 |
| Hydroxyapatite | Ca10(OH)2(PO4)6 |
| Magnetite | Fe3O4 |
| Noselite | 4Na2O3Al2O36SiO2SO4 |
| Pectolite | Na2O4CaO6SiO2H2O |
| Quartz | SiO2 |
| Serpentine | 3MgO2SiO22H2O |
| Thenardite | Na2SO4 |
| Wallastonite | CaSiO3 |
| Xonotlite | 5CaO5SiO2H2O |
Binding is often a function of surface charge and loss of water of hydration. Iron oxide, which exists in many hydrated and oxide forms, is particularly prone to bonding. Some silicates will do the same, and many oil contaminants are notorious deposit binders, due to polymerization and degradation reactions.
In addition to causing material damage by insulating the heat transfer path from the boiler flame to the water (Figure 12-2), deposits restrict boiler water circulation. They roughen the tube surface and increase the drag coefficient in the boiler circuit. Reduced circulation in a generating tube contributes to accelerated deposition, overheating, and premature steam-water separation.
Figures 12-3 and 12-4 illustrate the process of boiler circulation. The left legs of the U-tubes represent downcomers and are filled with relatively cool water. The right legs represent generating tubes and are heated. The heat generates steam bubbles, and convection currents create circulation. As more heat is applied, more steam is generated and the circulation rate increases.
If deposits form (Figure 12-4), the roughened surface and partially restricted opening resist flow, reducing circulation. At a constant heat input the same amount of steam is generated, so the steam-water ratio in the generating tube is increased. The water in the tube becomes more concentrated, increasing the potential for deposition of boiler water salts.
In extreme cases, deposition becomes heavy enough to reduce circulation to a point at which premature steam-water separation occurs. When this happens in a furnace tube, failure due to overheating is rapid. When deposits are light they may not cause tube failures, but they reduce any safety margin in the boiler design.
Up to the point of premature steam-water separation, the circulation rate of a boiler is increased with increased heat input. Often, as illustrated in Figure 12-5, the inflection point (A) is above the nominal boiler rating. When the circuit is dirty, the inflection point of the circulation-to-heat input curve moves to the left, and the overall water circulation is reduced. This is represented by the lower broken line.
Circulation and deposition are closely related. The deposition of particles is a function of water sweep as well as surface charge (Figure 12-6). If the surface charge on a particle is relatively neutral in its tendency to cause the particle either to adhere to the tube wall or to remain suspended, an adequate water sweep will keep it off the tube. If the circulation through a circuit is not adequate to provide sufficient water sweep, the neutral particle may adhere to the tube. In cases of extremely low circulation, total evaporation can occur and normally soluble sodium salts deposit.
Sodium carbonate treatment was the original method of controlling calcium sulfate scale. Today's methods are based on the use of phosphates and chelants. The former is a precipitating program, the latter a solubilizing program.
Carbonate Control
Before the acceptance of phosphate treatment in the 1930's, calcium sulfate scaling was a major boiler problem. Sodium carbonate treatment was used to precipitate calcium as calcium carbonate to prevent the formation of calcium sulfate. The driving force for the formation of calcium carbonate was the maintenance of a high concentration of carbonate ion in the boiler water. Even where this was accomplished, major scaling by calcium carbonate was common. As boiler pressures and heat transfer rates slowly rose, the calcium carbonate scale became unacceptable, as it led to tube overheating and failure.
Phosphate Control
Calcium phosphate is virtually insoluble in boiler water. Even small levels of phosphate can be maintained to ensure the precipitation of calcium phosphate in the bulk boiler water-away from heating surfaces. Therefore, the introduction of phosphate treatment eliminated the problem of calcium carbonate scale. When calcium phosphate is formed in boiler water of sufficient alkalinity (pH 11.0-12.0), a particle with a relatively nonadherent surface charge is produced. This does not prevent the development of deposit accumulations over time, but the deposits can be controlled reasonably well by blowdown.
In a phosphate precipitation treatment program, the magnesium portion of the hardness contamination is precipitated preferentially as magnesium silicate. If silica is not present, the magnesium will precipitate as magnesium hydroxide. If insufficient boiler water alkalinity is being maintained, magnesium can combine with phosphate. Magnesium phosphate has a surface charge that can cause it to adhere to tube surfaces and then collect other solids. For this reason, alkalinity is an important part of a phosphate precipitation program.
The magnesium silicate formed in a precipitating program is not particularly adherent. However, it contributes to deposit buildup on a par with other contaminants. Analyses of typical boiler deposits show that magnesium silicate is present in roughly the same ratio to calcium phosphate as magnesium is to calcium in boiler feedwater.
Phosphate/Polymer Control
Phosphate treatment results are improved by organic supplements. Naturally occurring organics such as lignins, tannins, and starches were the first supplements used. The organics were added to promote the formation of a fluid sludge that would settle in the mud drum. Bottom blowdown from the mud drum removed the sludge.
There have been many advances in organic treatments (Figure 12-7). Synthetic polymers are now used widely, and the emphasis is on dispersion of particles rather than fluid sludge formation. Although this mechanism is quite complex, polymers alter the surface area and the surface charge to mass ratio of typical boiler solids. With proper polymer selection and application, the surface charge on the particle can be favorably altered (Figure 12-8).
Many synthetic polymers are used in phosphate precipitation programs. Most are effective in dispersing magnesium silicate and magnesium hydroxide as well as calcium phosphate. The polymers are usually low in molecular weight and have numerous active sites. Some polymers are used specifically for hardness salts or for iron; some are effective for a broad spectrum of ions. Figure 12-9 shows the relative performance of different polymers used for boiler water treatment.
Table 12-2. Phosphate/polymer performance can be maintained at high heat transfer rates through selectionof the appropriate polymer.
Chelant Control
Chelants are the prime additives in a solubilizing boiler water treatment program. Chelants have the ability to complex many cations (hardness and heavy metals under boiler water conditions). They accomplish this by locking metals into a soluble organic ring structure. The chelated cations do not deposit in the boiler. When applied with a dispersant, chelants produce clean waterside surfaces.
Suppliers and users of chelants have learned a great deal about their successful application since their introduction as a boiler feedwater treatment method in the early 1960's. Chelants were heralded as "miracle treatment" additives. However, as with any material, the greatest challenge was to understand the proper application.
Chelants are weak organic acids that are injected into boiler feedwater in the neutralized sodium salt form. The water hydrolyzes the chelant, producing an organic anion. The degree of hydrolysis is a function of pH; full hydrolysis requires a relatively high pH.
The anionic chelant has reactive sites that attract coordination sites on cations (hardness and heavy metal contaminants). Coordination sites are areas on the ion that are receptive to chemical bonding. For example, iron has six coordination sites, as does EDTA (ethylenediaminetetraacetic acid). Iron ions entering the boiler (e.g., as contamination from the condensate system) combine with EDTA. All coordination sites on the iron ion are used by the EDTA, and a stable metal chelate is formed (Figure 12-10).
NTA (nitrilotriacetic acid), another chelant applied to boiler feedwater, has four coordination sites and does not form as stable a complex as EDTA. With NTA, the unused coordination sites on the cation are susceptible to reactions with competing anions.
Chelants combine with cations that form deposits, such as calcium, magnesium, iron, and copper. The metal chelate formed is water-soluble. When the chelate is stable, precipitation does not occur. Although there are many substances having chelating properties, EDTA and NTA are, to date, the most suitable chelants for boiler feedwater treatment.
The logarithm of the equilibrium constant for the chelant-metal ion reaction, frequently called the Stability Constant (Ks ), can be used to assess the chemical stability of the complex formed. For the calcium-EDTA reaction:
(Ca-EDTA)2 Ks = log = 10.59
(Ca)2+ (EDTA)4
Table 12-3 lists stability constants for EDTA and NTA with common feedwater contaminants.
Table 12-3. Stability constants provide a measure of chemical stability of the chelant-metal ion complexes.
| Metal Ion |
EDTA |
NTA |
| Ca+2 | 10.59 | 6.41 |
| Mg+2 | 8.69 | 5.41 |
| Fe+2 | 14.33 | 8.82 |
| Fe+3 | 25.1 | 15.9 |
The effectiveness of a chelant program is limited by the concentration of the competing anions. With the exception of phosphate, the competing anion limitations on EDTA chelation are not usually severe. Alkalinity and silica, in addition to phosphate, are restricting considera-tions in the use of NTA.
Chelant/Polymer Control
Iron oxide is of particular concern in today's boiler water treatment programs. Deposition from low (less than 1.0 ppm) hardness boiler feedwater is eliminated with chelant programs and can be reduced by up to 95% by a good polymer/phosphate treatment program. Iron oxide is an increasingly significant contributor to boiler deposits because of the virtual elimination of hardness deposits in many systems and because the high heat transfer rates of many boilers encourage iron deposition.
Chelants with high stability values, such as EDTA, can complex iron deposits. However, this ability is limited by competition with hydrate ions. Experience has shown that relying on EDTA or other chelants alone is not the most satisfac-tory method for iron control.
At normal chelant feed rates, limited chelation of incoming particulate iron occurs. This is usually enough to solubilize some condensate iron contamination. The chelation of magnetite (the oxide formed under boiler conditions-a mix of Fe2O3 and FeO) is possible because the chelant combines with the ferrous (FeO) portion of the magnetite.
Overfeed (high levels) of chelant can remove large quantities of iron oxide. However, this is undesirable because high excess chelant cannot distinguish between the iron oxide that forms the protective magnetite coating and iron oxide that forms deposits.
A chelant/polymer combination is an effective approach to controlling iron oxide. Adequate chelant is fed to complex hardness and soluble iron, with a slight excess to solubilize iron contamination. Polymers are then added to condition and disperse any remaining iron oxide contamination (Figure 12-11).
A chelant/polymer program can produce clean waterside surfaces, contributing to much more reliable boiler operation (Figure 12-12). Out-of-service boiler cleaning schedules can be extended and, in some cases, eliminated. This depends on operational control and feedwater quality. Chelants with high complexing stabilities are "forgiving" treatments-they can remove deposits that form when feedwater quality or treatment control periodically deviates from standard.
Boilers with moderate deposition in the forms of calcium carbonate and calcium phosphate can be cleaned effectively through an in-service chelant cleanup program. In-service chelant cleanup programs should be controlled and not attempted on a heavily deposited boiler or applied at too fast a pace. Chelants can cause large accumulations of deposit to slough off in a short period of time. These accumulations can plug headers or redeposit in critical circulation areas, such as furnace wall tubes.
In a chelant cleanup program, sufficient chelant is added to solubilize incoming feedwater hardness and iron. This is followed by a recommended excess chelant feed. Regular inspections (usually every 90 days) are highly recommended so that the progress of the treatment may be monitored.
The polymer level in the boiler should also be increased above the normal concentration. This confines particles to the bulk water as much as possible until they settle in the mud drum. An increased number of mud drum "blows" should be performed to remove the particles from the boiler.
In-service chelant cleanup programs are not advisable when deposit analyses reveal that major constituents are composed of silicates, iron oxide, or any scale that appears to be hard, tightly bound, or lacking in porosity. Because such scales are not successfully removed in most in-stances, an in-service chelant cleanup cannot be justified in these situations.
Phosphate/Chelant/Polymer Combinations
Combinations of polymer, phosphate, and chelant are commonly used to produce results comparable to chelant/polymer treatment in low- to medium-pressure boilers. Boiler cleanliness is improved over phosphate treatment, and the presence of phosphate provides an easy means of testing to confirm the presence of treatment in the boiler water.
Polymer-Only Treatment
Polymer-only treatment programs are also used with a degree of success. In this treatment, the polymer is usually used as a weak chelant to complex the feedwater hardness. These treatments are most successful when feedwater hardness is consistently very low.
High-Pressure Boiler Water Treatment
High-pressure boilers usually have areas of high heat flux and feedwater, composed of demineralized makeup water and a high percentage of condensate returns. Because of these conditions, high-pressure boilers are prone to caustic attack. Low-pressure boilers that use demineralized water and condensate as feedwater are also susceptible to caustic attack.
There are several means by which boiler water can become highly concentrated. One of the most common is iron oxide deposition on radiant wall tubes. Iron oxide deposits are often quite porous and act as miniature boilers. Water is drawn into the iron oxide deposit. Heat applied to the deposit from the tube wall generates steam, which passes out through the deposit. More water enters the deposit, taking the place of the steam. This cycle is repeated and the water beneath the deposit is concentrated to extremely high levels. It is possible to have 100,000 ppm of caustic beneath the deposit while the bulk water contains only about 5-10 ppm of caustic (Figure 12-13).
Steam generating units supplied with demineralized or evaporated makeup water or pure condensate may be protected from caustic corrosion by a treatment known by the general term "coordinated phosphate/pH control." Phosphate is a pH buffer in this program and limits the localized concentration of caustic. A detailed discussion of this treatment is included in Chapter 11.
If deposits are minimized, the areas where caustic can be concentrated is reduced. To minimize the iron deposition in high-pressure (1000-1750 psig) boilers, specific polymers have been designed to disperse the iron and keep it in the bulk water.
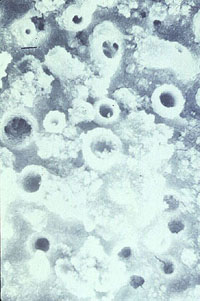
As with phosphate precipitation and chelant control programs, the use of these polymers with coordinated phosphate/pH treatment improves deposit control. Figure 12-14 illustrates the effectiveness of dispersants in controlling iron oxide deposition. Test conditions were 1500 psig (590°F), 240,000 Btu/ft²/hr heat flux, and coordinated phosphate/pH program water chemistry. A comparison of the untreated heat transfer surface (shown at left) with the polymer dispersant treated conditions (shown at right) provides a graphic illustration of the value of dispersants in preventing steam generator deposition. The ability to reduce iron oxide accumulations is an important requirement in the treatment of boiler systems operating at high pressures and with high-purity feedwater.
Supercritical boilers use all-volatile treatments, generally consisting of ammonia and hydrazine. Because of the extreme potential for deposit formation and steam contamination, no solids can be tolerated in supercritical once-through boiler water, including treatment solids.
Figure 12-1. Classification of Deposits.
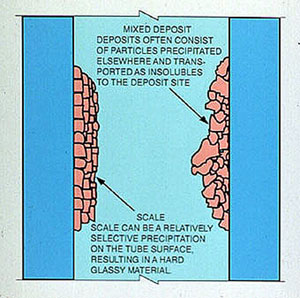
Figure 12-2. Deposition reduces heat transfer from boiler tube to boiler water, increasing the tube metal temperature. Tube metal overheating and failure can occur.
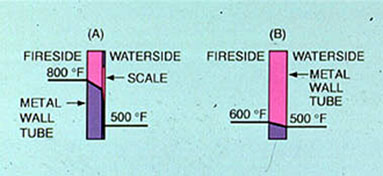
Figure 12-4. U-tube illustrates water circulation and steam generation with deposits.
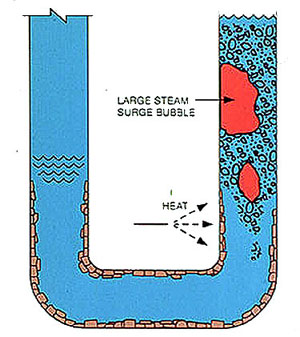
Figure 12-5. Circulation as a function of heat input in a boiler circuit.
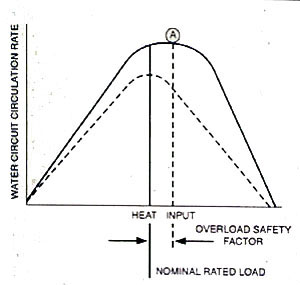
Figure 12-6. Opposing forces act on water-carried particles. Surface charges may attract particles to a deposit. Water flow "sweeps" the particle along.
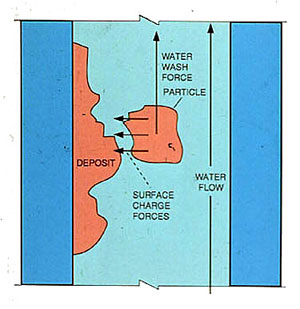
Figure 12-7. Experimental boilers are used to evaluate chemical treatment programs under rigorous conditions.

Figure 12-8. (Left) Scanning electron photomicrograph (4000X magnification) of calcium phosphate-magnesium silicate crystals formed in boiler water not treated with dispersant. (Right) With a sulfonated polymer, crystal growth is controlled.
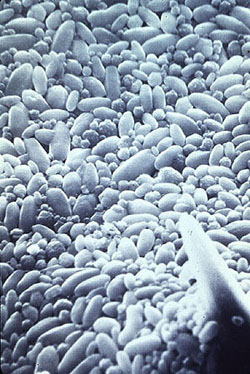
Figure 12-9. Although many polymers are available for boiler water treatment application, performance levels vary.
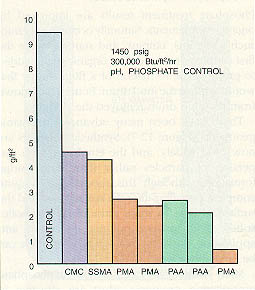
Figure 12-10. Most metals have six reactive coordination sites. EDTA can effectively tie into each coordination site and produce a stable complex.
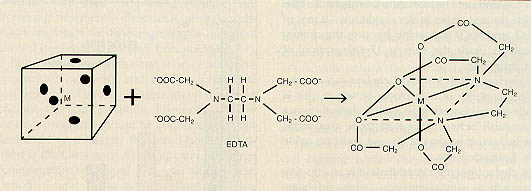
Figure 12-11. Chelant/polymer can provide a high degree of iron deposit protection, provided that the proper polymer is used. Even the members of the same family of polymers, such as polymethacrylate (PMA), can vary greatly in performance.
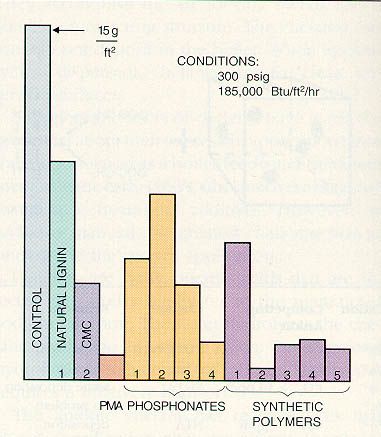
Table 12-2. Phosphate/polymer performance can be maintained at high heat transfer rates through selection of the appropriate polymer.
| Treatment Type | Boiler Treatment Concentration (ppm) | Heat Transfer Rate (Btu/ft2/hr) | Operating Pressure (psig) | % Scale Reduction |
| Synthetic Polymer A | 10 | 185,000 | 300 | 44 |
| Synthetic Polymer B | 10 | 185,000 | 300 | 93 |
| Synthetic Polymer C | 10 | 185,000 | 300 | 94 |
| Synthetic Polymer B | 5 | 185,000 | 300 | 56 |
| Synthetic Polymer C | 5 | 185,000 | 300 | 94 |
| Synthetic Polymer B | 10 | 185,000 | 900 | 64 |
| Synthetic Polymer C | 10 | 185,000 | 900 | 92 |
| Synthetic Polymer B | 10 | 300,000 | 900 | 44 |
| Synthetic Polymer C | 10 | 300,000 | 900 | 86 |
| Synthetic Polymer B | 10 | 300,000 | 1200 | 30 |
| Synthetic Polymer C | 10 | 240,000 | 1200 | 90 |
| Synthetic Polymer C | 10 | 300,000 | 1200 | 83 |
Figure 12-12. Chelant/polymer provides the most deposit-free mode of internal treatment control. Test conditions: 600 psig; 60,000 (large probe) + 180,000 (small probe) Btu/ft2/hr feedwater, makeup constant.
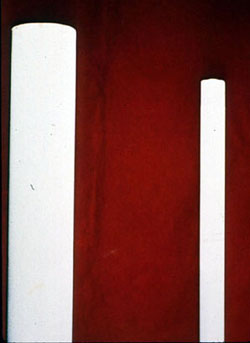 Phosphate Cycle--No Treatment |
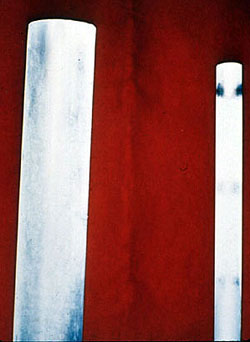 Phosphate Cycle--Natural Conditioner |
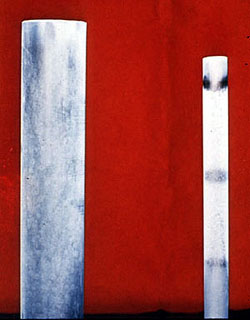 Phosphate Cycle--Lignin Conditioner |
 Phosphate Cycle--Polymeric Dispersants |
 Phosphate Cycle--Chelant and Polymer Dispersant Blend |
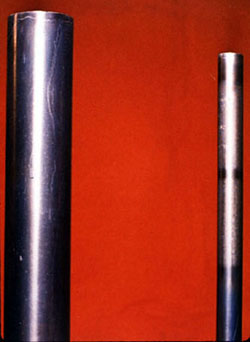 Chelation Cycle--Chelant and Polymer Dispersant Blend |
Figure 12-13. Porous deposits provide conditions that promote high concentrations of boiler water solids, such as sodium hydroxide (NaOH).
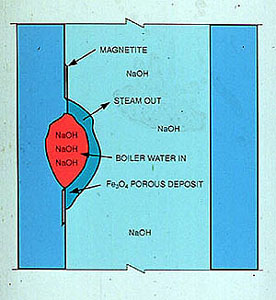
Figure 12-14. Experimental boiler heat transfer suraces (800X magnifications) exposed to feedwater iron contamination. Heavy iron oxide deposition occurred (left) when no polymer was used. A virtually clean surace was achieved with an iron-specific polymer program (right).