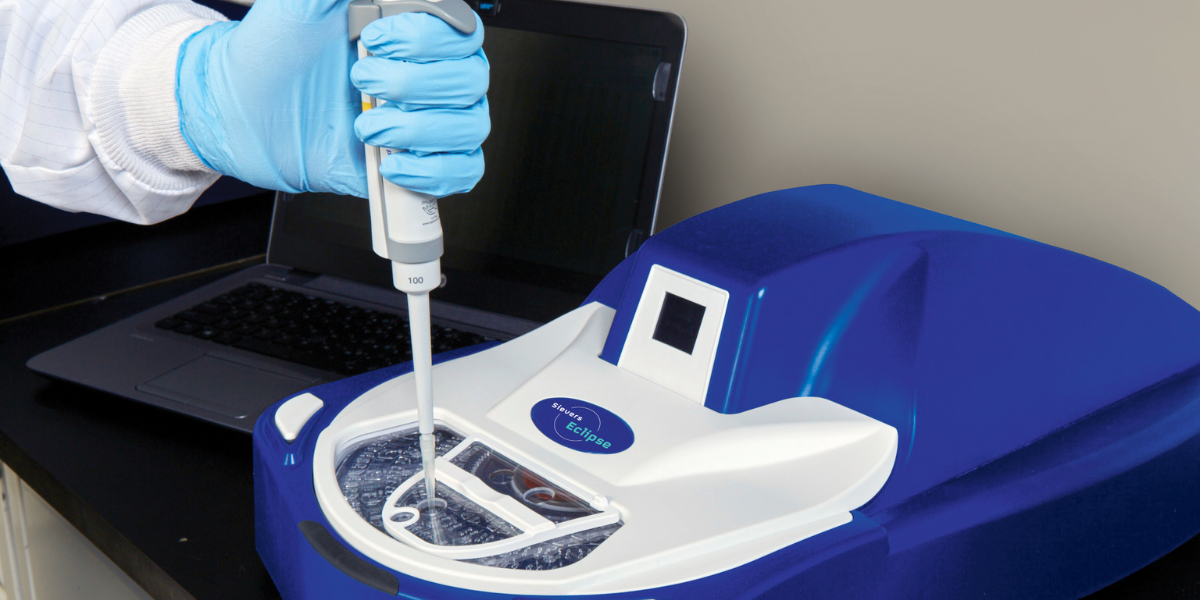
Simplified Quality Control (QC) testing is a must in pharmaceutical and medical device manufacturing today. The Sievers Eclipse BET Platform not only offers simplification, but it also offers compliance and a significant reduction in the use of Limulus Amebocyte Lysate (LAL) and other resources. Learn why analysts all over the world are switching to the Eclipse and reaping the benefits of improved QC testing and simplification using centripetal microfluidics for BET.
- The Sievers Eclipse is user friendly and fully compliant with all compendia including requirements of USP <85>, EP 2.6.14, and JP 4.01.
- The Sievers Eclipse runs a minimum 3-point standard curve in at least duplicate using standardized endotoxin . Pre-loaded standards and PPCs save time and reduce pipetting Negative controls are in at least duplicate
- The Eclipse reduces opportunity for contamination and errors using an enclosed system, automation, and significantly fewer pipetting steps.
- The Sievers Eclipse minimizes LAL usage by up to 90% compared to traditional assays.
- You can increase and improve operational efficiency with the ability to run multiple plates in just a few hours and run 63 samples with just one (1) vial of LAL reagent.
- The Sievers Eclipse enterprise software is fully compliant with 21 CFR Part 11 and ALCOA+ data integrity guidelines. And it’s easy to use, includes assay-specific audit trails, and offers electronic signature for remote sign off.
- Training and validation are easy and streamlined - validation can be completed within a few days, and you can train an analyst in just a few hours.
The Sievers Eclipse BET platform can greatly improve the efficiency of your BET processes. Simplify Your BET testing today.
- Author:
-
Author: Meg Provenzano Life Sciences Product Manager of Bio-Detection, Veolia | Water Tech, Sievers Instruments
She has over 10 years of experience in the bacterial endotoxin testing industry and has held several positions in Quality Control, Technical Support, and Product Management. She is customer-centric and enjoys hands-on problem solving, whether for technical issues, assay assistance, or software. Meg holds a B.S. in Marine Science and Biology from Coastal Carolina University where she focused on Bottlenose Dolphin population research.
