
Pharmaceutical manufacturers rely on total organic carbon (TOC) measurements as one of the compendial requirements for release of water and equipment for production. Accurate and consistent TOC analysis is critical to meet the acceptance criteria limits for USP regulations.
Did you know there is a difference between sensors vs analyzers for TOC testing?
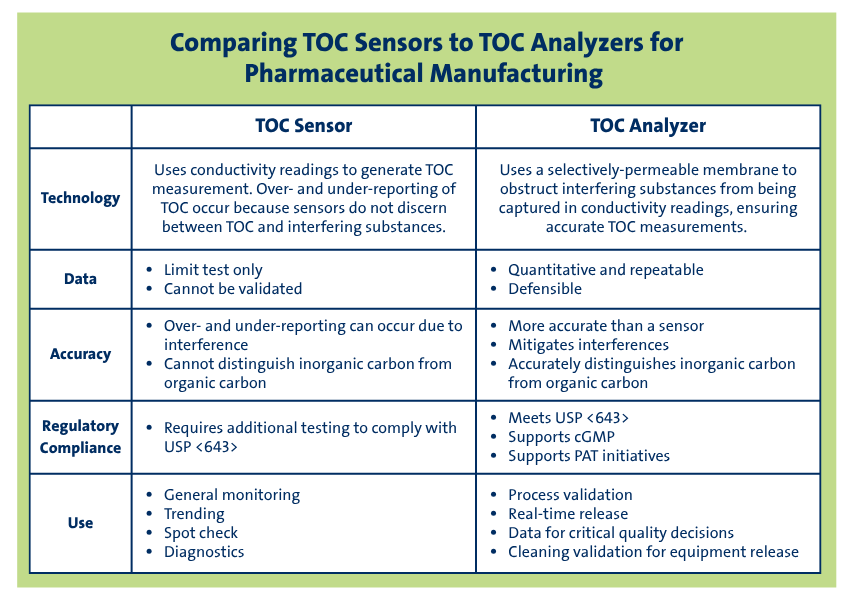
Sensors are more commonly limited to monitoring applications (trending, diagnostics, or general monitoring), whereas analyzers are leveraged for applications that require process control and reporting (process validation, quality management, real-time release, cleaning validation, etc.). To satisfy regulatory requirements and ensure a reliable manufacturing process, it is essential for users to understand the differences between these two types of instruments.
Use of TOC in pharmaceutical applications
In pharmaceutical and biopharmaceutical manufacturing, TOC instruments serve as critical tools for ultrapure water testing/compliance monitoring, as well as validating or verifying cleaning samples. Choosing the right analytical instrument is important, and the selection depends on the pharmaceutical manufacturers’ needs and the characteristics of the process or samples they need to measure.
While TOC sensors are more cost effective and accommodate faster response time, the ability of analyzers to report on critical quality decisions for compliance may serve as a better solution for many applications. Manufacturers seeking to implement process analytical technology (PAT) to the full capability of real-time release will be seeking a validated instrument that satisfies instrument qualification, method validation, and data integrity needs.
Technology Comparison: TOC Sensors
TOC sensors work by employing conductivity as a means to measure carbon; however they do not discriminate against interfering ions that may be contributing to the measurements. These technologies measure conductivity in a sample pre- and post-oxidation and derive the TOC result from the assumption that all measured conductivity post-oxidation arises from organic carbon conversion to CO2.
This can lead to over- and under-reporting of TOC.
Additionally, there is no differentiation between CO2 from inorganic carbon (IC) and organic carbon, as required by USP <643>[1]. Sensors can effectively pass the limit test, but because methods cannot be validated[2] during performance qualification for specificity and robustness, actual analytical performance for organic compounds is widely variable. Proper method validation is imperative in cGMP manufacturing settings, especially considering that pharmaceutical grade water touches many phases in the manufacturing process. TOC sensors are often selected for general monitoring applications, or as portable tools for trending.
TOC Analyzers
TOC analyzers, on the other hand, are carbon specific analyzers, wherein a gas-permeable membrane separates CO2 from interfering compounds to allow for accurate measurement of carbon. The selectivity of the membrane-based analyzers provides better accuracy for TOC measurements and makes these instruments most appropriate for compendial applications, cleaning validation, process control, and regulatory reporting. This technology accurately and precisely measures both organic and inorganic carbon as required by USP <643>, allowing for successful performance qualification and effective analysis for specificity and robustness compounds.
Benefits of TOC Analyzers
TOC analyzers offer precise, accurate, quantitative, and robust carbon analysis, giving manufacturers the ability to pass performance qualification and validation, thus providing users with an accurate assessment of the state of the water system. With validated and accurate results, data can be used to make important quality decisions for pharmacopeia compliance, release water and equipment, troubleshoot, and optimize water monitoring and cleaning processes.
Another benefit is avoiding under-reported values; this helps mitigate the risk of infecting byproducts and other harmful compounds that are present in water systems which could place the final product at risk of contamination. On the other hand, over-reporting TOC can cause unnecessary and time-consuming out-of-specification (OOS) investigations where no definitive root cause is identified.
Additional in-depth comparison
Comparing TOC analyzers and sensors for pharmaceutical applications reveals critical performance differences, supported by scientific evidence. While direct conductometric sensors are capable of detecting organic compounds, this study demonstrated significant accuracy issues (i.e., erroneously high, low, and non-linear responses) across multiple compound types:
- For chlorinated organic compounds, sensors showed variable recovery (some 150% recovery, or greater), whereas membrane-based analyzers were much closer to 100% recovery
- For a nitrogen-containing compound like nicotinamide, sensors showed ~150% recovery due to the formation of nitric acid during oxidation, while membrane-based analyzers maintained ~100% recovery
- For conductive organic compounds (trimethyl amine and acetic acid), sensors produced unreliable results (less than 50%, or negative recovery) compared to membrane-based analyzers' consistent ~100% recovery
These findings align with third-party research that emphasizes, “robust analytical TOC method validation is essential to the success of any online TOC system, particularly systems that release pharmaceutical grade water in real time. Meeting USP <643> or EP 2.2.44 specifications may not eliminate risk.”
Robust method validation is critical since sensors might not eliminate risks, particularly for real-time release of pharmaceutical-grade water. In contrast, the superior selectivity and consistent accuracy of membrane-based conductometric analyzers make them optimal for compendial applications, process control, cleaning verification, and reporting on critical quality decisions.
Download the “Comparison of TOC Analyzers and Sensors for Pharmaceutical TOC Applications” Application Note to explore the full study.
Conclusion
With TOC sensor technology, there can be risks to product safety due to over- or under-reporting of TOC. Sensors do not differentiate between CO2 from inorganic carbon (IC) and organic carbon, as required by USP <643>.
TOC analyzers use membrane-based conductivity to mitigate interferences experienced with TOC sensors, improving accuracy and precision. Analyzers are ideal for applications that require process control and reporting for pharmacopoeia compliance. TOC analyzers are available in portable, bench-top, or online configurations, allowing manufacturers to choose what fits their application best. TOC analyzers are more complex instruments that can handle multiple sample points and perform more detailed analysis with greater accuracy and additional parameters. Additionally, fully validated and compliant TOC analyzers can be deployed for PAT applications, supporting real-time monitoring and release. Learn more about the Sievers Instruments product line and what TOC instrument is right for your processes here.
- References
