Since the 1940's, ion exchange resins have been used to remove dissolved salts from water. These resins exchange ions in the water for ions on the resin exchange sites and hold them until released by a regeneration solution (see Chapter 8 for a more detailed discussion). Many ion exchange processes exist for a variety of industrial water and wastewater applications. The ion exchange process consumes large quantities of regeneration chemicals, such as brine, acid, and caustic materials that can present significant handling and disposal problems.
In recent years, membrane processes have been used increasingly for the production of "pure" waters from fresh water and seawater. Membrane processes are also being applied in process and wastewater systems.
Although thought to be expensive and relatively experimental in the past, membrane technology is advancing quickly becoming less expensive, improving performance, and extending life expectancy.
Common membrane processes include ultrafiltration (UF), reverse osmosis (RO), electrodialysis (ED), and electrodialysis reversal (EDR). These processes (with the exception of UF) reduce most ions; RO and UF systems also provide efficient reduction of nonionized organics and particulates. Because UF membrane porosity is too large for ion rejection, the UF process is used to reduce contaminants, such as oil and grease, and suspended solids.
Reverse Osmosis
Osmosis is the flow of solvent through a semi-permeable membrane, from a dilute solution to a concentrated solution. This flow results from the driving force created by the difference in pressure between the two solutions. Osmotic pressure is the pressure that must be added to the concentrated solution side in order to stop the solvent flow through the membrane. Reverse osmosis is the process of reversing the flow, forcing water through a membrane from a concentrated solution to a dilute solution to produce filtered water. Figure 9-1 illustrates the processes of osmosis and reverse osmosis.
Reverse osmosis is created when sufficient pressure is applied to the concentrated solution to overcome the osmotic pressure. This pressure is provided by feedwater pumps. Concentrated contaminants (brine) are reduced from the high-pressure side of the RO membrane, and filtered water (permeate) is reduced from the low-pressure side. Figure 9-2 is a simplified schematic of an RO process. Membrane modules may be staged in various design configurations, producing the highest-quality permeate with the least amount of waste. An example of a multistage RO configuration is shown in Figure 9-3.
Typically, 95% of dissolved salts are reduced from the brine. All particulates are removed. However, due to their molecular porosity, RO membranes do not remove dissolved gases, such as Cl2, CO2, and O2.
RO Membranes. The two most common RO membranes used in industrial water treatment are cellulose acetate (CA) and polyamide (PA) composite. Currently, most membranes are spiral wound; however, hollow fiber configurations are available. In the spiral wound configuration, a flat sheet membrane and spacers are wound around the permeate collection tube to produce flow channels for permeate and feedwater. This design maximizes flow while minimizing the membrane module size.
Hollow fiber systems are bundles of tiny, hair-like membrane tubes. Ions are rejected when the feedwater permeates the walls of these tubes, and permeate is collected through the hollow center of the fibers. Concentrated brine is produced on the outside of the fibers contained by the module housing.
Figure 9-4 shows the construction and flow patterns in a spiral wound membrane configuration.Figure 9-5 shows the construction and flow patterns in a hollow fiber membrane system.
Electrodialysis
Electrodialysis (ED) processes transfer ions of dissolved salts across membranes, leaving purified water behind. Ion movement is induced by direct current electrical fields. A negative elec-trode (cathode) attracts cations, and a positive electrode (anode) attracts anions. Systems are compartmentalized in stacks by alternating cation and anion transfer membranes. Alternating compartments carry concentrated brine and filtered permeate. Typically, 40-60% of dissolved ions are removed or rejected. Further improvement in water quality is obtained by staging (operation of stacks in series). ED processes do not remove particulate contaminants or weakly ionized contaminants, such as silica. Figure 9-6 is a simplified schematic of an ED process.
Electrodialysis Reversal
Electrodialysis reversal (EDR) processes operate on the same principles as ED; however, EDR operation reverses system polarity (typically 3-4 times per hour). This reversal stops the buildup of concentrated solutions on the membrane and thereby reduces the accumulation of inorganic and organic deposition on the membrane surface. EDR systems are similar to ED systems, designed with adequate chamber area to collect both product water and brine. EDR produces water of the same quality as ED.
Ultrafiltration
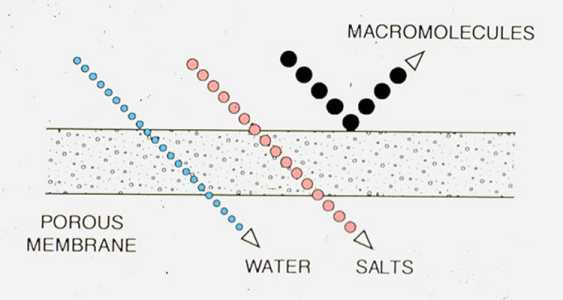
In many process and wastewater applications, reduction of dissolved ions is not required but efficient reduction of colloidal inorganic or organic molecules is. Ultrafiltration (UF) membrane configurations and system designs are similar to those used in the single-stage RO process. Because the large molecules removed by UF exhibit negligible osmotic pressure, operating pressures are usually much lower than in RO systems. Figure 9-7 illustrates the performance of ultrafiltration membranes. Typical applications include reduction of oil and grease and recovery of valuable contaminants in process waste streams.
Processes that rely on microporous membranes must be protected from fouling. Membrane foul-ing causes a loss of water production (flux), reduced permeate quality, and increased trans-membrane pressure drop.
Membrane fouling is typically caused by precipitation of inorganic salts, particulates of metal oxides, colloidal silt, and the accumulation or growth of microbiological organisms on the membrane surface. These fouling problems can lead to serious damage and necessitate more frequent replacement of membranes.
Membrane feedwater should be relatively free from colloidal particulates. The most common particulates encountered in industrial membrane systems are silt, iron oxides, and manganese oxides.
Silt Density Index (SDI) testing should be used to confirm sufficient water quality for the specific membrane system employed. SDI evaluates the potential of feedwater to foul a 0.45 µm filter. Unacceptable SDI measurements can be produced even when water quality is relatively high by most industrial water treatment standards. Where pretreatment is inadequate or ineffective, chemical dispersants may be used to permit operation at higher-than-recommended SDI values. RO systems are highly susceptible to particulate fouling, ED and EDR systems are more forgiving, and UF systems are designed to handle dirty waters.
Membrane processes produce a concentration gradient of dissolved salts approaching the membrane surfaces. The concentration at the membrane may exceed the solubility limits of certain species. Calcium carbonate (CaCO3) and calcium sulfate (CaSO4) are typical precipitates formed. Silica, barium, and strontium salts are also frequently identified in membrane deposits. Because of their low solubility, very low levels of feedwater barium or strontium can cause membrane fouling.
Various saturation indexes, such as the Stiff-Davis and Langelier, should be maintained below precipitating values in the brine (through pH control or deposit control agents) to prevent calcium carbonate fouling. Other precipitates may be controlled by the proper application of deposit control agents.
Cellulose acetate membranes can be degraded by microbiological activity. Proper maintenance of chlorine residuals can prevent microbiological attack of these membranes.
Polyacrylamide membranes are resistant to microbiological degradation; however, they are susceptible to chemical oxidation. Therefore, chlorination is not an acceptable treatment. If inoculation occurs, microbiological fouling can become a problem. Nonoxidizing antimicrobials and biodispersants should be used if serious microbiological fouling potential exists.
Learn more about how Veolia's membrane protection chemical products can protect your membranes from scale, bacterial growth and fouling.
Figure 9-1. In the osmosis process, water flows through a membrane from the dilute solution side tohe more concentrated solution side. In reverse osmosis, applied pressure causes water to flow from the concentrated solution to the dilute solution.
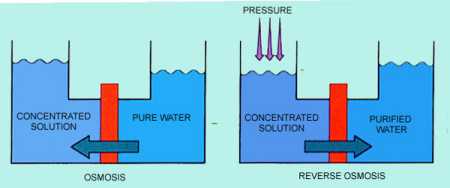
Figure 9-2. A reverse osmosis system converts a feed stream into a purified stream (permeate) and a concentrated stream (brine).
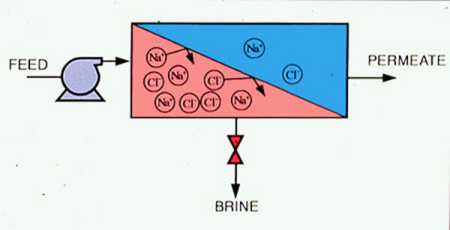
Figure 9-3. A multistage reverse osmosis system configured to reduce the quantity of waste brine.
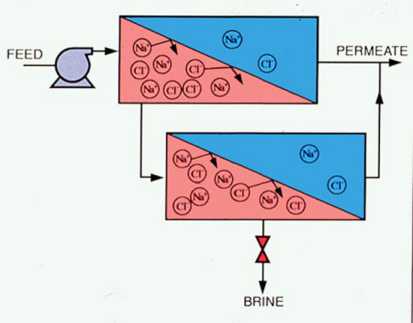
Figure 9-4. Spiral wound reverse osmosis modules are widely used. (Reprinted with permission from McGraw Hill, "Standard Handbook of Environmental Engineering.")
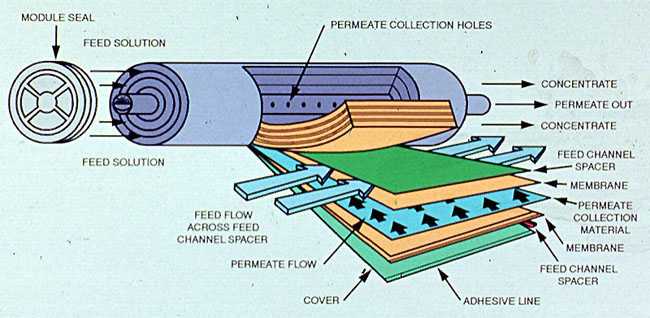
Figure 9-5. Hollow fiber reverse osmosis modules.
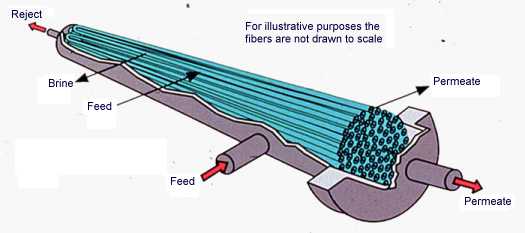
Figure 9-6. In electrodialysis and electrodialysis reversal ions pass through alternating cation and anion transfer membranes.
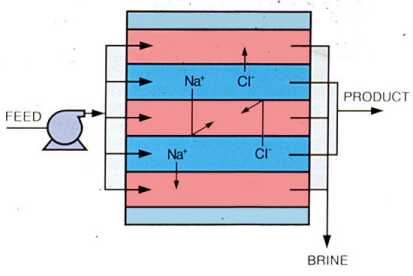
Figure 9-7. Ultrafiltration membranes pass inorganic ions but reject large organic molecules and colloidal particles.