- Corrosion of iron
- Corrosion of copper
- Effect of pH on corrosion of iron and copper
- Effect of other contaminants
- Chemical treatment of condensate systems
Problems caused by iron and copper corrosion in condensate systems are not restricted to piping and equipment damage or to the loss of high-quality water and heat energy when condensate is lost. If returned to the boiler, corrosion products and process chemicals from corrosion-caused leaks contribute to the formation of damaging boiler deposits, boiler carryover, and steam-driven equipment deposits. Their presence reduces system reliability and increases operation and maintenance costs.
Iron corrodes in water even in the absence of oxygen. An iron oxide surface acts like a car battery, with the surface divided into microscopic anodes (+) and cathodes (-). In condensate systems, iron acts as an anode so that it is oxidized (i.e., gives its electrons to the cathode). The cathode in pure water is a proton or hydrogen ion (H+). When iron metal is oxidized, electrons are passed from the iron surface to hydrogen ions as shown in the reactions below.
Oxidation:
| Fe | « |
Fe2+ |
+ | 2e |
| iron | ferrous ion | electrons |
Reduction:
| 2H+ | + |
2e |
« | H2 |
| hydrogenion | electrons | hydrogengas |
Overall:
|
Fe |
+ | 2H+ | « | Fe2+ | + | H2 |
|
iron |
hydrogenion |
ferrousion |
hydrogen gas |
The fate of the ferrous ion (Fe2+) depends on condensate temperature, pH, and flow conditions. At low temperatures, Fe2+ reacts with water to form insoluble ferrous hydroxide, Fe(OH)2. If the condensate stream velocity is high enough, colloidal Fe(OH)2 is swept into the water and car-ried downstream to deposit elsewhere. In low-flow areas of the condensate system, Fe(OH)2deposits near the oxidation site, forming a porous oxide layer.
At temperatures above 120°F the deposited ferrous hydroxide reacts further to form surface-bound magnetite (Fe3O4) crystals.
| 3Fe(OH)2 | « | Fe3O4 | + | 2H2O | + | H2 |
|
ferrous hydroxide |
magnetite |
water |
hydrogen gas |
At even higher temperatures (above 300°F), Fe2+ spontaneously forms magnetite without first forming Fe(OH)2. This magnetite forms a nonporous, tightly adherent layer on the metal surface.
| 3Fe2+ | + | 4H2O | « | Fe3O4 | + | 4H2 |
|
ferrous ion |
|
water |
|
magnetite |
|
hydrogen gas |
In most condensate systems, two or three forms of iron oxide are present. In pure water, a tightly adherent magnetite layer is formed, which is indicative of a well passivated iron surface. In the absence of contaminants, this oxide layer greatly retards any further oxidation reactions.
Oxygen Corrosion of Iron
In the presence of oxygen, the corrosion process described above is modified. Dissolved oxygen replaces hydrogen ions in the reduction reaction. The reactions are as follows:
Oxidation:
| Fe | « |
Fe2+ |
+ | 2e |
|
iron |
|
ferrous ion |
|
electrons |
Reduction:
| O2 | + |
2e |
« | O2 |
|
oxygen |
|
electrons |
|
oxide ion |
Overall:
| Fe | + | O2 | + | 2H+ | « | Fe2+ | + | H2O |
|
iron |
|
oxygen |
|
hydrogen ion |
|
ferrous ion |
|
water |
This reaction occurs more readily than the direct reaction between iron and protons. Therefore, corrosion rates are accelerated in the presence of oxygen.
Two types of corrosion can occur with oxygen present. The first, generalized corrosion on the metal surface, causes a loss of metal from the entire surface. The second, oxygen pitting (Figure 19-1), causes a highly localized loss of metal that results in catastrophic failure in a short time.
Oxygen pitting begins at weak points in the iron oxide film or at sites where the oxide film is damaged. Instead of growing along the metal surface, the corrosion penetrates into the surface, effectively drilling a hole into (or through) the metal.
Pits are active only in the presence of oxygen. There is a visible difference between active and inactive pits. An active oxygen pit contains reduced black oxide along its concave surface, while the surrounding area above the pit is covered with red ferric oxide. If a pit contains red iron oxide, it is no longer active.
Sources of Oxygen. Oxygen usually enters the condensate by direct absorption of air. It can also flash over with the steam when the feedwater contains oxygen. With effective mechanical deaeration and chemical oxygen scavenging, all but a trace of oxygen is eliminated from boiler feedwater, so this source is not significant in most systems.
In a good system design, the air-condensate contact is minimized to prevent oxygen absorption. The condensate receiving tank can be designed with a cover to reduce air contact and a steam heating coil within the tank to elevate condensate temperature and thereby reduce oxygen solubility.
Under certain conditions, gross oxygen contamination of the condensate may be unavoidable. For example, condensate from warm-up steam for equipment used only intermittently should not be saved. Its dissolved oxygen attacks systems between the point of condensation and the deaerating heater. This contaminated condensate can return large amounts of corrosion products to the boiler.
In most cases, proper feedwater deaeration and elimination of air infiltration into the condensate substantially reduce oxygen corrosion.
Although copper is similar to iron in chemistry, the form of the resulting oxide layer is very different. Both copper and iron are oxidized in the presence of hydrogen ions and oxygen and can undergo oxygen pitting.
| 2Cu | + | 2H+ | « | 2Cu+ | + | H2 |
| copper | hydrogen ion | cuprous ion | hydrogen gas |
or in alkaline solution:
| 2Cu | + | H2O | « | Cu2O | + | H2 |
| copper | water | cuprous oxide | hydrogen gas |
Iron forms intact oxide layers. The oxide layers formed by copper and its alloys are porous and "leaky," allowing water, oxygen, and copper ions to move to and from the metal surface (Figure 19-2).
The rate of movement is controlled by the copper oxide film thickness. As the oxide layer grows in thickness, the copper oxidation rate is slowed. As the oxide layer becomes thicker, the outer layers begin to slough off as particles of copper oxide. The resulting oxide layer is a much more dynamic system than that of iron. Soluble copper ions and particulate copper oxides are also formed by the normal oxidation processes.
EFFECT OF pH ON CORROSION OF IRON AND COPPER
The stability of the passivating iron or copper oxide layer is critically dependent on condensate pH. Any contaminants in the condensate system that cause the pH to decrease cause dissolution of the oxide layer and increased corrosion.
Carbon dioxide (CO2) is the primary cause of decreased condensate pH. Carbon dioxide enters the system with air leaking into the condenser or from decomposition of feedwater alkalinity. Although part of the feedwater alkalinity is removed by a properly operated deaerating heater, most is converted to CO2 in the boiler and released into the steam.
In boilers, carbon dioxide is liberated as shown by the following reactions:
| 2NaHCO3 | + | heat | « | Na2CO3 | + | CO2 | + | H2O |
|
sodium bicarbonate |
|
|
|
sodium carbonate |
|
carbon dioxide |
|
water |
|
Na2CO3 |
+ |
H2O |
+ |
heat |
« |
2NaOH |
+ |
CO2 |
|
sodium carbonate |
|
water |
|
|
|
sodium hydroxide |
|
carbon |
The first reaction proceeds to completion while the second is only (approximately) 80% completed. The net results are release of 0.79 ppm of carbon dioxide for each part per million of sodium bicarbonate as CaCO3 and 0.35 ppm of carbon dioxide for each part per million of sodium carbonate as CaCO3.
As the steam is condensed, carbon dioxide dis-solves in water and depresses the pH by increasing the hydrogen ion concentration as shown in the following reaction sequence:
|
CO2 |
+ |
H2O |
« |
H2CO3 |
|
carbon dioxide |
|
water |
|
carbonic acid |
|
H2CO3 |
« |
H+ |
+ |
HCO3 |
|
carbonic acid |
|
hydrogen ion |
|
bicarbonate ion |
|
HCO3 |
« |
H+ |
+ |
CO32 |
|
bicarbonate ion |
|
hydrogen ion |
|
carbonate ion |
Carbonic acid promotes the iron corrosion re-action by supplying a reactant, H+. The overall reaction is:
|
2H+ |
+ |
2HCO3 |
+ |
Fe |
« |
Fe(HCO3)2 |
+ |
H2 |
|
hydrogen ion |
|
bicarbonate ion |
|
iron |
|
ferrous bicarbonate |
|
hydrogen |
Low pH causes a generalized loss of metal rather than the localized pitting caused by oxygen corrosion. Pipe walls are thinned, particularly in the bottom of the pipe. This thinning often leads to failures, especially at threaded sections (Figure 19-3).
In order to reduce low pH-induced condensate system corrosion, it is necessary to lower the concentration of acidic contaminants in the condensate. Feedwater alkalinity can be reduced by means of various external treatment methods. Less feedwater alkalinity means less carbon dioxide in the steam and condensate. Venting at certain points of condensation can also be effective in removing carbon dioxide.
Other contaminants in the condensate system can affect corrosion rates of iron and copper even when the pH is correctly maintained. By complexing and dissolving iron and copper oxides, contaminants such as chloride, sulfide, acetate, and ammonia (for copper) can dissolve part or all of the oxide layer.
Ammonia is the most common contaminant and is usually present in low concentrations. Ammonia contamination is usually caused by the breakdown of nitrogenous organic contaminants, hydrazine, or amine treatment chemicals. Sometimes, ammonia is fed to control condensate pH. In these systems, ammonia feed rates must be carefully controlled to minimize the attack of any copper-bearing alloys (Figure 19-4).
CHEMICAL TREATMENT OF CONDENSATE SYSTEMS
Condensate systems can be chemically treated to reduce metal corrosion. Treatment chemicals include neutralizing amines, filming amines, and oxygen scavenger-metal passivators.
Neutralizing Amines
Neutralizing amines are used to neutralize the acid (H+) generated by the dissolution of carbon dioxide or other acidic process contaminants in the condensate. These amines hydrolyze when added to water and generate the hydroxide ions required for neutralization:
|
R-NH2 |
+ |
H2O |
« |
R-NH3+ |
+ |
OH |
|
neutralizing amine |
|
water |
|
amine ion |
|
hydroxide ion |
The overall neutralization reaction can be written as shown:
|
R-NH3+ |
+ |
OH |
+ |
H2CO3 |
« |
R-NH3+ |
+ |
HCO3 |
+ |
H2O |
|
amine ion |
|
hydroxide ion |
|
carbonic acid |
|
amminium ion |
|
bicarbonate ion |
|
water |
By regulating the neutralizing amine feed rate, the condensate pH can be elevated to within a desired range (e.g., 8.8-9.2 for a mixed copper-iron condensate system).
Many amines are used for condensate acid neutralization and pH elevation. The ability of any amine to protect a system effectively depends on the neutralizing capacity, recycle ratio and recovery ratio, basicity, distribution ratio, and thermal stability of the particular amine.
Neutralizing Capacity. Neutralizing capacity is the concentration of acidic contaminants that is neutralized by a given concentration of amine. The neutralizing capacity of an amine is inversely proportional to molecular weight (i.e., lower molecular weight yields higher neutralizing capacity) and directly proportional to the number of amine groups. Neutralizing capacity is important in treating systems with high-alkalinity feedwater. Table 19-1 provides a measure of the neutralizing capacity of commonly employed amines. Neutralizing capacity is not the only measure of a required product feed rate.
Table 19-1. Relative neutralizing capacities
| Amine | ppm Neutralizing Amine/ppm CO2 |
| Cyclohexylamine | 2.3 |
| Morpholine | 2.0 |
| Diethylaminoethanol | 2.6 |
| Dimethlyisopropanolamine | 2.3 |
Recycle Ratio and Recovery Ratio. In determining product feed rates, recycle and recovery ratio are important factors. In Figure 19-5, the recycle factor is the concentration of amine at point x divided by the amine feed rate at point z. Because some amine is returned with the condensate, the total amine in the system is greater than the amount being fed. Recovery ratio is a measure of the amount of amine being returned with the condensate. It is calculated as the amine concentration at site y divided by the amine con-centration at site z.
Basicity. An amine's ability to boost pH after neutralizing all of the acid species is termed "basicity." In chemical terms, it is a measure of an amine's ability to hydrolyze in pure water. The dissociation constant Kb is a common measure of basicity.
|
Kb = |
[R-NH3+] [OH] |
| [R-NH2] |
As the value of Kb increases, more OH is formed (after all of the acid has been neutralized) and pH increases.
Examples of neutralizing amine Kb values at various temperatures are provided in Table 19-2.
Table 19-2. Relative basicity
| Relative Basicity KbX 106 |
|||
| Amine | 72 °F | 298 °F | 338 °F |
| Cyclohexylamine | 489 | 61 | 32 |
| Morpholine | 3.4 | 4.9 | 3.8 |
| Diethylaminoethanol | 68 | 11.3 | 9.2 |
| Dimethlyisopropanolamine | 20.6 | 6.9 | 4.6 |
Distribution of Amines between Steam and Liquid. In condensate systems, the distribution of amines between steam and liquid phases is as significant as basicity or neutralizing capacity. As the steam condenses, acidic contaminants can either remain in the steam or dissolve in the liquid phase. Some contaminants, such as carbon dioxide, stay mainly in the steam phase while others, such as hydrochloric acid, go largely into the liquid phase.
Neutralizing amines must be chosen according to their distribution characteristics to "chase" acidic contaminants. This choice must be tailored to the condensate system and the process contaminants.
For example, morpholine is an amine that primarily distributes into the liquid phase. If this amine is fed into a CO2 laden steam system with three consecutive condensation sites, it will go into the liquid phase at the first condensation site while most of the carbon dioxide remains in the steam. With a high concentration of morpholine, the liquid phase has a high pH. At the next condensation site, the concentration of morpholine is lower, so the condensate pH is lower. At the last condensation site, where the remaining steam is condensed, little morpholine is left but most of the CO2 is still present. The high CO2 concentration depresses the pH, promoting acidic attack of the metal oxide layers.
An amine that is more likely to distribute into the steam, such as cyclohexylamine, is a better choice for the system described above. However, amines with a high tendency to distribute into the steam are not always the best choice.
For example, if cyclohexylamine is used in a second condensate system with two consecutive condensation sites having acetic acid as a contaminant, most of the acetic acid goes into the liquid phase at the first condensation site, while most of the cyclohexylamine remains in the steam. This results in low pH in the first condensation site liquid phase. At the second site, where total condensation takes place, the pH is high. A morpholine-cyclohexylamine blend is a better choice for this system.
In practice, the best protection is provided by blended products containing a variety of amines with differing steam/liquid distributions.
To compare the relative steam/liquid distribution of amines, the distribution ratio (DR) is traditionally used. The distribution ratio of an amine is:
|
DR = |
amine in vapor phase |
|
amine in liquid phase |
Amines with a DR greater than 1.0 produce a higher concentration of amine in the vapor phase than in the liquid phase. Conversely, amines with a distribution ratio less than 1.0 produce a higher concentration of amine in the liquid phase than in the vapor phase.
Distribution ratios are not true physical constants but are a function of pressure (Figure 19-6) and pH. The effect of temperature and pH of the condensation site must also be considered. In a complex condensate system, the distribution of chemicals between different condensation sites is difficult to estimate without the use of computer modeling packages specifically designed for this purpose.
Thermal Stability. All organic chemicals exposed to a high-temperature, alkaline, aqueous environment eventually degrade to some degree. Most amines eventually degrade to carbon dioxide and/or acetic acid and ammonia. The time required varies greatly with different amines. The most stable amines commonly used are morpholine and cyclohexylamine. These remain substantially intact at pressures up to 2500 psig.
Quantity. The quantity of neutralizing amine required depends on the carbon dioxide content of the condensate at specific locations and the degree of corrosion protection desired. Complete neutralization is achieved if the condensate pH in all portions of the system is above 8.3. From a practical standpoint, it is necessary to establish a pH control range that provides the desired protection for the most critical system components.
The degree of protection can be monitored by various means. Corrosion test specimens installed in bypass racks, corrosion product analyses, corrosion rate meters, and submicron corrosion product filtration are some of the effective monitoring tools that may be employed.
The behavior of amine bicarbonate in the deaerator affects amine requirements for the system. Although they are soluble in most cases, amine bicarbonates remain associated in the condensate. In an ideal situation, the amine bicarbonate entering the deaerator breaks down with subsequent venting of carbon dioxide to the atmosphere and recirculation of the amine back to the boiler. Actual behavior involves some loss of amine additive and some recirculation of carbon dioxide. The amounts of lost amine and retained carbon dioxide are a function of the amine bicarbonate stability in the deaerator.
Filming Amines
Another approach to controlling condensate system corrosion is the use of chemicals that form a protective film on metal surfaces (Figure 19-7). This approach has come into widespread use with the development of suitable products containing long-chain nitrogenous materials.
Filming amines protect against oxygen and carbon dioxide corrosion by replacing the loose oxide scale on metal surfaces with a very thin amine film barrier. During the period of initial film formation, old, loosely adherent corrosion products are lifted off the metal surface due to the surfactant properties of the amine. The metal is cleansed of oxides, which normally cling very tightly and can build up over long periods of time. Excessive initial filming amine treatment of old, untreated or poorly treated condensate systems can cause large amounts of iron oxide to be sloughed off, plugging traps and return lines. Therefore, treatment must be increased gradually for old systems.
When contaminants are present in the condensate, filming amines have a tendency to form deposits by reacting with multivalent ions, such as sulfate, hardness, and iron. Overfeed of filming amines and excessive oxygen contamination can also contribute to deposit formation. For maximum efficiency, filming amines should be fed directly to the steam header.
Advances have been made in formulating filming amine treatments. Straight filming amines containing one ingredient, such as octadecylamine, are effective but often fail to cover the entire system and can produce fouling. Emulsifiers and, in some cases, small amounts of neutralizing amines can be added to improve film distribution by providing more uniform coverage. This increases system protection and reduces the fouling potential. Application experience has shown that combination amines (filming and neutralizing amines with dispersant aids) provide a superior film bond, reduce deposit problems, and provide better system coverage and thus provide more complete and economical corrosion protection (Figure 19-8).
The feed of filming amines is usually based on steam throughput. Different levels of treatment are required, depending on the particular blend in use. As in the case of neutralizing amines, various methods are used to monitor the effectiveness of the treatment, including corrosion test specimens installed in bypass coupon racks (Figure 19-9), iron analyses, corrosion rate meters, and submicron corrosion product filtration.
Oxygen Scavenging and Metal Passivation
Where oxygen invades the condensate system, corrosion of iron and copper-bearing components can be overcome through proper pH control and the injection of an oxygen scavenger.
One important factor to consider in choosing an oxygen scavenger for condensate treatment is its reactivity with oxygen at the temperature and pH of the system. A scavenger that removes oxygen rapidly provides the best protection for the condensate metallurgy. Hydroquinone has been shown to be particularly effective for most systems.
Like those of neutralizing amines, the steam/liquid distribution of each scavenger has a unique temperature dependence. Some scavengers, such as ascorbic acid and hydrazine, have a very low volatility. Therefore, it is necessary to feed them close to a problem area. An example of this is the injection of hydrazine to the exhaust of a turbine to protect the condenser. Other scavengers, such as hydroquinone, are relatively volatile and can be fed well upstream of a problem area.
The use of neutralizing amines in conjunction with an oxygen scavenger/metal passivator improves corrosion control in two ways. First, because any acidic species present is neutralized and pH is increased, the condensate becomes less corrosive. Second, most oxygen scavenger/passivators react more rapidly at the mildly alkaline conditions maintained by the amine than at lower pH levels. For these reasons, this combination treatment is gaining wide acceptance, particularly for the treatment of condensate systems that are contaminated by oxygen.
Figure 19-1. Typical oxygen pitting of condensate line.
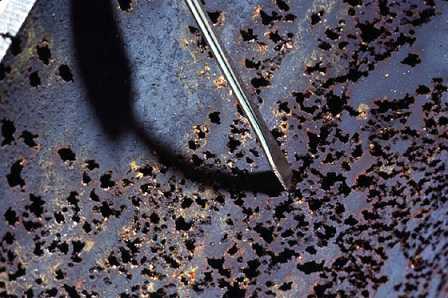
Figure 19-2. Unlike protective magnetite layers, copper oxide layers are porous and allow water, oxygen, and copper ions to move to and from the metal surface.
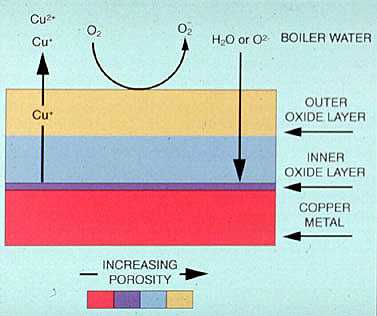
Figure 19-3. Section of condensate line destroyed by carbon dioxide (low pH) corrosion. Metal destruction is spread over a relatively wide area, resultingin thinning.

Figure 19-4. The protective copper oxide film can be destroyed by complexing agents, such as ammonia.
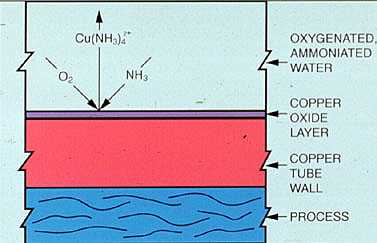
Figure 19-5. Because a portion of the amine feed is recycled, the amine concentration in the system usually exceeds the feed rate.
Figure 19-6. Graph shows how the distribution ratios of cyclohexylamine and diethylaminoethanol vary with pressure.
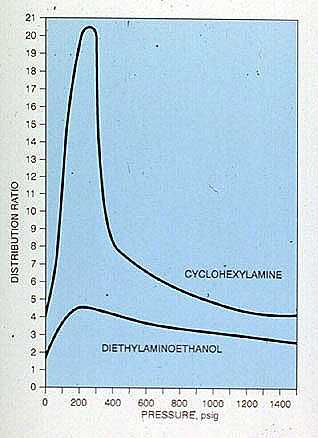
Figure 19-7. Test specimen 381 shows the nonwettable surface produced by an effective filming amine. Specimen 380 is untreated.
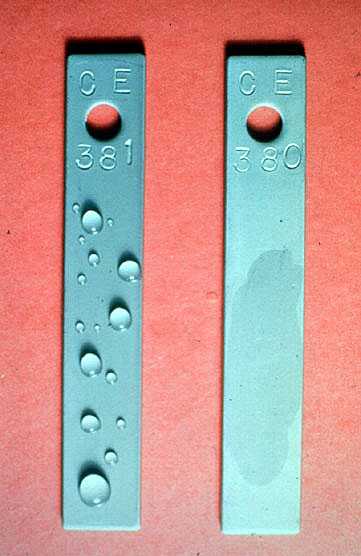
Figure 19-8. Feedwater iron determination is one method of monitoring the effectiveness of an amine treatment program. The application of a combination of a neutralizing -filming amine treatment reduced feedwater iron from 0.5 ppm to 0.05 ppm in just 2 months.
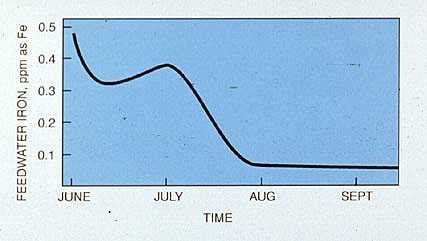
Figure 19-9. Test specimen bypass rack used to monitor amine treatment. The corrosion rate meter on the right measures relative corrosiveness instantaneously.

