Veolia's microbiological control agents can help treat and protect cooling systems from a variety of micro-organisms and microbiological growth.
- Difficulties due to Biofouling
- Microbiology of Cooling Water
- Biofilms
- Choice of Slime Control Program
- Characteristics of Nonoxidizing Chemicals Used in Cooling Systems
- Proprietary Nonoxidizing Chemicals
Cooling water systems, particularly open recirculating systems, provide a favorable environment for the growth of microorganisms. Microbial growth on wetted surfaces leads to the formation of biofilms. If uncontrolled, such films cause fouling, which can adversely affect equipment performance, promote metal corrosion, and accelerate wood deterioration. These problems can be controlled through proper biomonitoring and application of appropriate cooling water antimicrobials.
DIFFICULTIES DUE TO BIOFOULING
Microbiological fouling in cooling systems is the result of abundant growth of algae, fungi, and bacteria on surfaces. Once-through and open or closed recirculating water systems may support microbial growth, but fouling problems usually develop more quickly and are more extensive in open recirculating systems.
Once-through cooling water streams generally contain relatively low levels of the nutrients essential for microbial growth, so growth is relatively slow. Open recirculating systems scrub microbes from the air and, through evaporation, concentrate nutrients present in makeup water. As a result, microbe growth is more rapid. Process leaks may contribute further to the nutrient load of the cooling water. Reuse of wastewater for cooling adds nutrients and also contributes large amounts of microbes to the cooling system.
In addition to the availability of organic and inorganic nutrients, factors such as temperature, normal pH control range, and continuous aeration of the cooling water contribute to an environment that is ideal for microbial growth. Sunlight necessary for growth of algae may also be present. As a result, large, varied microbial populations may develop.
The outcome of uncontrolled microbial growth on surfaces is "slime" formation. Slimes typically are aggregates of biological and nonbiological materials. The biological component, known as the biofilm, consists of microbial cells and their by-products. The predominant by-product, extracellular polymeric substance (EPS), is a mixture of hydrated polymers. These polymers form a gel-like network around the cells and appear to aid attachment to surfaces. The nonbiological components can be organic or inorganic debris from many sources which have become adsorbed to or embedded in the biofilm polymer.
Slimes can form throughout once-through and recirculating systems and may be seen or felt where accessible. In nonexposed areas, slimes can be manifested by decreased heat transfer efficiency or reduced water flow. Wood-destroying organisms may penetrate the timbers of the cooling tower, digesting the wood and causing collapse of the structure. Microbial activity under deposits or within slimes can accelerate corrosion rates and even perforate heat exchanger surfaces.
Microorganisms
The microorganisms that form slime deposits in cooling water systems are common soil, aquatic, and airborne microbes (see Figure 26-1). These microbes may enter the system with makeup water, either in low numbers from fresh water sources or in high numbers when the makeup is wastewater. Significant amounts may also be scrubbed from the air as it is drawn through the cooling tower. Process leaks may contribute microorganisms as well.
Bacteria. A wide variety of bacteria can colonize cooling systems. Spherical, rod-shaped, spiral, and filamentous forms are common. Some produce spores to survive adverse environmental conditions such as dry periods or high temperatures. Both aerobic bacteria (which thrive in oxygenated waters) and anaerobic bacteria (which are inhibited or killed by oxygen) can be found in cooling systems.
Fungi. Two forms of fungi commonly encountered are molds (filamentous forms) and yeasts (unicellular forms). Molds can be quite troublesome, causing white rot or brown rot of the cooling tower wood, depending on whether they are cellulolytic (attack cellulose) or lignin degrading. Yeasts are also cellulolytic. They can produce slime in abundant amounts and preferentially colonize wood surfaces.
Algae. Algae are photosynthetic organisms. Green and blue-green algae are very common in cooling systems (blue-green algae are now classified with the bacteria and are called cyanobacteria). Various types of algae can be responsible for green growths which block screens and distribution decks. Severe algae fouling can ultimately lead to unbalanced water flow and reduced cooling tower efficiency. Diatoms (algae enclosed by a silicaceous cell wall) may also be present but generally do not play a significant role in cooling system problems.
Differences and Similarities
Although algae, fungi, and bacteria differ in many respects, they also share many characteristics. These similarities and differences are important in understanding biofouling and its control.
- Cell size differs according to the complexity of the cell structure. The simpler bacteria and cyanobacteria are much smaller than molds, yeasts, and other algae. Because of their faster metabolisms and rates of growth, these smaller cells are able to reproduce much more rapidly.
- All microorganisms require water for growth. Although they vary in terms of absolute water requirements and ability to survive dry periods, an active, viable microbial population cannot exist without water.
- Most microorganisms growing in cooling systems are bound by a rigid cell wall. The cell wall gives the organism its characteristic shape and provides mechanical strength. Immediately inside the cell wall is a cell membrane which functions as a permeability barrier for the cell. The barrier allows the cell to concentrate desirable chemicals, such as nutrients, and exclude or excrete toxic or unwanted chemicals, such as waste materials. Concentration gradients of several orders of magnitude can be established across the membrane. All cells must expend considerable amounts of metabolic energy to maintain an optimal interior condition. One essential characteristic of all microbes is the ability to preserve the necessary organization and integrity of the cell in a hostile and changing environment.
- All cells must obtain energy and chemical "building blocks" from their environment in order to survive and grow. The ability of each type of cell to fulfill this function in different environments is discussed in the following section.
Microbiological Growth
Among the essential building blocks used by microbial cells, and those needed in largest quantity, are carbon, nitrogen, and phosphorus. Microbes differ in the method they use to obtain carbon. Green algae, cyanobacteria, and certain bacteria can utilize carbon dioxide as a sole carbon source and convert ("fix") it to cellular carbon compounds. Most bacteria, yeast, and molds require preformed carbon compounds and use organic molecules that range from very simple to very complex. In order to meet nitrogen requirements, microbes "fix" atmospheric nitrogen or utilize amines, nitrites, and nitrates present in the environment. Naturally occurring and synthetic inorganic and organic phosphates can be used to meet microbial phosphate requirements.
Microbes have developed many ways to extract energy from their surroundings. Algae and other photosynthetic organisms trap light energy from the sun. Inorganic chemicals, such as ammonia, sulfur, and hydrogen, can be oxidized by certain bacteria to release energy. More commonly, bacteria, yeasts, and molds liberate chemical energy stored in organic compounds, such as sugars, proteins, fats, oils, organic acids, and alcohols.
Aerobic organisms use oxygen to drive the oxidations that release chemical energy. Anaerobes do not use oxygen but may substitute molecules such as sulfate or nitrate in place of oxygen. In the anaerobic energy-yielding process, these oxidizing molecules are reduced, forming sulfides or nitrogen gas. When no acceptable oxidizer is available, some anaerobes can still generate energy, although less efficiently, by coupling oxidation of one half of a substrate molecule to reduction of the other half. Typically, the by-products of this "fermentative" reaction are various organic acids. All microbes extract and collect energy in small, usable packets. Once the energy is made available, there are only minor differences in how it is used.
In the presence of sufficient nutrients, growth and reproduction can occur. Bacteria and cyanobacteria multiply by binary fission, a process in which a cell divides to form two identical daughter cells. Yeasts divide by budding, with a mother cell repeatedly forming single, identical but much smaller daughter cells. Filamentous molds grow by forming new cells at the growing tip of the filament. Green algae can have several patterns of growth, depending on the species, ranging from tip extension to production of several cells from a single cell during one division cycle. As with other cell features, the complexity of growth processes also increases with increasing cell size. Under optimal conditions, some bacteria can double their numbers every 20 to 30 minutes, while molds can take many hours to double in mass.
Microorganisms are also extremely adaptable to changes in their environment. This characteristic is related to cell size and complexity. The simple forms with minimal growth needs and fast growth rates can form many cell generations within a few days. Slight random changes in cellular characteristics during those generations can produce a new cell that is more capable of surviving in a shifted environment. This new cell can soon dominate the environment. Many microbes carry information in unexpressed form for functions to be performed when needed for survival. Changes in the environment can activate this information causing all members of a microbial population to achieve new capabilities as a group, within a single generation.
Usually, cooling waters are not nutrient-rich, so microbes must expend a great deal of energy transporting and concentrating nutrients inside the cell. This process may spend energy resources already in short supply, but it is necessary to allow the biochemical machinery to run at top speed. Because there is strong competition for the available nutrients, those species most efficient at concentrating their essential nutrients will have the opportunity to grow most rapidly. The rate of growth will ultimately be limited by the nutrient which first falls below an optimal concentration, but this will not necessarily be the nutrient in the lowest concentration.
Chemicals applied to cooling systems may, at times, provide added sources of the limiting nutrient and thus contribute to microbial growth in the systems. Alterations of pH may shift a stable population balance to an unbalanced, troublesome state. Although bacteria may be under control at neutral pH, a shift to an acid pH may result in domination by molds or yeast. Because many algae grow most abundantly at an alkaline pH, an attempt to reduce corrosion by raising the pH can lead to an algal bloom.
Seasonal changes also affect growth patterns in cooling water systems. Natural algal communities in a fresh water supply are quite dynamic, and the dominant species can change rapidly with changing temperatures, nutrients, and amounts of sunlight. Cyanobacteria can often be primary colonizers in a cooling system. Seasonal changes which increase their numbers in the makeup water can lead to an algal bloom in the system. In autumn, as falling leaves increase the nutrient level and depress the pH, the bacterial population can increase at the expense of the algal population.
Microbiologists recognize two different populations of microorganisms. Free-floating (planktonic) populations are found in the bulk water. Attached (sessile) populations colonize surfaces. The same kinds of microorganisms can be found in either population, but the sessile population is responsible for biofouling.
Much is known about the formation of biofilms on wetted surfaces such as heat exchanger tubes. Microorganisms on submerged surfaces secrete polymers (predominantly polysaccharides but also proteins), which adhere firmly even to clean surfaces and prevent cells from being swept away by the normal flow of cooling water. These extracellular polymeric substances are hydrated in the natural state, forming a gel-like network around sessile microorganisms. This polymer network contributes to the integrity of the biofilm and acts as a physical barrier hindering toxic materials and predatory organisms from reaching the living cell (see Figure 26-2). Biofilm polymers can also consume oxidizers before they reach and destroy microorganisms. As a result, control of sessile microorganisms requires dosages many times greater than required to control planktonic organisms.
Biofilms develop slowly at first, because only a few organisms can attach, survive, grow, and multiply. As populations increase exponentially, the depth of the biofilm increases rapidly. Biofilm polymers are sticky and aid in the attachment of new cells to the colonized surface as well as the accumulation of nonliving debris from the bulk water. Such debris may consist of various inorganic chemical precipitates, organic flocs, and dead cell masses. Fouling results from these accumulative processes, along with the growth and replication of cells already on the surface and the generation of additional polymeric material by these cells.
When fouling occurs, even mechanical cleaning does not remove all traces of the biofilm. Previously fouled and cleaned surfaces are more rapidly colonized than new surfaces. Residual biofilm materials promote colonization and reduce the lag time before significant fouling reappears.
Biofilms on heat exchange surfaces act as insulating barriers. Heat exchanger performance begins to deteriorate as soon as biofilm thickness exceeds that of the laminar flow region. Microbes and hydrated biopolymers contain large amounts of water, and biofilms can be over 90% water by weight. As a result, biofilms have thermal conductivities very close to that of water and, in terms of heat transfer efficiency, a biofilm is the equivalent of a layer of stagnant water along the heat exchange surface.
In shell-and-tube heat exchangers, the resistance to heat transfer is least in the turbulent flow of the bulk phase, slightly greater across the metal tube walls, and greatest across laminar flow regions. As biofilm thickness increases, so does the apparent thickness of the laminar flow region. Like water, biofilms are 25 to 600 times more resistant to conductive heat transfer than many metals. A small increase in the apparent thickness of the laminar region due to biofilm growth has a significant impact on heat transfer. A thin biofilm reduces heat transfer by an amount equal to a large increase in exchanger tube wall thickness. For example, the resistance to heat transfer of a 1 mm thick accumulation of biofilm on a low carbon steel exchanger wall is equivalent to an 80 mm increase in tube wall thickness.
Biofilms can promote corrosion of fouled metal surfaces in a variety of ways (see Figure 26-3). This is referred to as microbially influenced corrosion (MIC) and is discussed further in Chapter 25. Microbes act as biological catalysts promoting conventional corrosion mechanisms:
- the simple, passive presence of the biological deposit prevents corrosion inhibitors from reaching and passivating the fouled surface
- microbial reactions can accelerate ongoing corrosion reactions
- microbial by-products can be directly aggressive to the metal
The physical presence of a biofilm and biochemical activity within the film change the environment at the fouled surface. Differences between colonized and uncolonized sites may promote a galvanic-like attack. Microbes consume oxygen more rapidly than it can be transferred from the bulk solution, and areas beneath the biofilm become anaerobic and anodic. Repassivation of colonized surfaces is also hindered. Some microbes deprived of oxygen switch to fermentative metabolisms and produce large amounts of organic acids. This can result in local areas of low pH. Growth of anaerobes, such as sulfate-reducing bacteria, is favored in low-oxygen environments. These bacteria can oxidize hydrogen forming at the cathode and depolarize the corrosion cell. Their sulfide by-products may be directly corrosive or may contribute further to the electrochemical differential between fouled and unfouled sites.
In summary, microbes originating in the natural environment colonize cooling systems by capitalizing on favorable environmental conditions. Cooling systems are favorable environments for microorganisms because they contain water, operate in acceptable temperature and pH ranges, and provide nutrients for growth. Microbial attachment to surfaces in untreated systems produces deposits which reduce equipment efficiency and can be highly destructive to cooling equipment.
CHOICE OF SLIME CONTROL PROGRAM
Because of the speed with which microbes can grow in cooling water systems, frequent monitoring of these systems is essential for the identification of developing problems. Vigilant monitoring of operating data can identify trends, and periodic system inspections show whether or not fouling is occurring. Test coupons and test heat exchangers may be used in operating systems to facilitate monitoring without interrupting system operation.
Deposits collected from the cooling system can be analyzed in the laboratory to determine their chemical composition and biological content. If a deposit has a significant microbiological content, its causative agents should be identified for treatment. The laboratory can identify the agents as predominantly algal, bacterial, or fungal, either microscopically (see Figure 26-4) or by routine cultural isolation and identification.
Microbial counts can also be performed to determine whether populations within the system are stable, increasing, or decreasing. Usually, planktonic populations are monitored by means of the Standard Methods plate count technique. However, not all organisms in the fouling process can be detected by this method. Anaerobic bacteria, such as sulfate-reducers which can cause under-deposit corrosion, are not revealed by aerobic cultural procedures. Special techniques must be used to ensure detection of these organisms (see Figures 26-5 and 26-6). Figure 26-5. Figure 26-6.
Sole reliance on bulk water counts will not provide sufficient information on the extent of surface fouling. Results must be interpreted in light of operating conditions at the time of sample collection. For example, in an untreated system a healthy, stable biofilm population may be present while bulk water counts are low, because few sessile organisms are being released from the fouled surface. If an antimicrobial is applied, bulk water counts may actually increase dramatically. This is due to disruption of the biofilm and sloughing of sessile organisms into the bulk water.
For a better diagnosis, it is necessary to use microbial monitoring techniques that allow more direct assessment of surface conditions. It is possible to clean a known surface area and suspend removed organisms in a known volume of sterile water. After this water is plated, back-calculation provides an approximation of the number of organisms on the original surface.
Another technique involves monitoring biochemical activity on a surface of a known area. A biofouled specimen is incubated with a suitable substrate. The concentration of reaction product found after a specific contact time relates to the numbers and health of organisms on the surface and consequently can be used as a measure of biofouling.
Regardless of which target population or monitoring technique is used, a single, isolated data point has little meaning. Various data must be compiled to generate a profile of microbiological trends in the system. This record should also include observations on equipment performance and operating conditions at the time of sample collection, thereby providing a meaningful context for interpretation of new data.
After it is determined that treatment is necessary to solve a fouling problem, an effective product must be chosen. Preliminary choices may be made only if the causative microbial agent is known, because the spectrum of activity of all antimicrobials is not the same. Some effectively control algae but not bacteria. For others, the reverse is true. For some, the activity spectrum, determined by inhibition of radiolabeled nutrient uptake, is quite broad, covering all common cooling water microbes (see Table 26-1 and Figure 26-7).
Table 26-1. Antimicrobial efficacy.
|
I50 (ppm)* |
||||||||
| Bacteria | Fungi | Algae | Cyanobacteria | |||||
| Antimicrobial | Klebsiella pneumoniae | Bacillus megaterium | Candida krusei | Trichoderma viride | Chlorella Pyrenoidosa | Scenedesmus obliquus | Anacystis nidulans | Anabaena flos-aquae |
| Methylenebis-(thiocyanate) | 2.7 | 1.5 | 3.1 | 0.7 | 1.4 | 1.2 | 1.0 | 2.5 |
| Dibromoni-trilopropion-amide | 5.1 | 1.2 | 16 | 10 | 11 | 20 | 1.1 | 1.5 |
| B-Bromo-B-nitrostyrene | 3.6 | 2.5 | 2.0 | 0.6 | 0.85 | 5.0 | 1.5 | 0.7 |
| bromonitro-propanediol | 15 | 8.0 | -- | 27 | 80 | 120 | -- | -- |
Knowledge of how different antimicrobials affect microorganisms is also useful in choosing the appropriate treatment. Some kill the organisms they contact. Others inhibit growth of organisms but do not necessarily kill them. These biostats can be effective if a suitable concentration is maintained in a system for a sufficient time (a continuous concentration is ideal).
A laboratory evaluation of the relative effectiveness of antimicrobials should be performed. This helps to identify those likely to work against the fouling organisms in the system and to eliminate those with little chance of success. Because the goal of antimicrobial treatment is control or elimination of biofilm organisms, it is helpful to conduct the evaluation with sessile organisms found in deposits, as well as planktonic organisms in the flowing water.
The objective of any treatment program should be to expose the attached microbial population to an antimicrobial dosage sufficient to penetrate and disrupt the biofilm. Generally, cleanup of a fouled system requires higher concentrations of intermittently fed treatment, while maintenance of a clean system can be achieved with low-level continuous or semicontinuous feed. Given a certain level of fouling, the shorter the exposure time allowed by system operating conditions, the higher the required antimicrobial concentration. Conversely, if exposure times are long, control of the same level of fouling may be achieved with lower antimicrobial dosages.
Antimicrobial test results are most relevant when based on contact times derived from the system which is to be treated. Because once-through contact times are typically short, it can be very difficult to simulate these systems in lab testing. The longer contact times associated with recirculating cooling systems are easily duplicated in the lab.
In once-through systems, antimicrobials should be fed continuously to achieve the necessary contact time. Often, only low levels of antimicrobial are affordable on a continuous-feed basis. Semicontinuous treatment may be more economical or may be required because of effluent restrictions. Such an intermittent program for once-through systems must still be designed to achieve an effective antimicrobial concentration throughout the system, using treatment periods which range from minutes to hours per day.
Recirculating systems can also be treated continuously or intermittently, although intermittent treatment programs are more common. The purpose of intermittent treatment in these systems is to generate a high concentration of antimicrobial which will penetrate and disrupt the biofilm and eventually dissipate. When the treatment level drops below the toxic threshold, microbial growth begins again. After a period of multiplication, new growth is removed with another shock dose. As stated earlier, previously fouled surfaces can be recolonized at an accelerated rate. Therefore, the period for growth and removal may vary within a system, and certainly will vary among systems, even those using the same water source.
The time at which an antimicrobial concentra-tion drops below a threshold concentration in a recirculating system can be determined mathematically. Such information can be very useful in planning a schedule for effective and economical slime control. The threshold concentration desired should be estimated from a toxicant evaluation. The theoretical depletion of the antimicrobial from a system can be determined from the following formula:
BD x T Log Cf = log Ci - 2.303V where: Cf = final concentration, ppm Ci = initial concentration, ppm
BD = blowdown and windage loss, gpm V = system capacity, gal T = time, min
It is standard practice to repeat the shock treatment when Cf is 25% of Ci. On this basis, the time interval for antimicrobial addition can be calculated as follows:
V T = 1.385 x BD
Solving this equation for T will indicate how frequently a slug should be added to the system, but this determination is only valid for 75% depletion or two half-lives.
The equations provided are not valid for the following compounds:
- compounds which are volatile and may be lost during passage over the tower
- compounds which react with substances in the water (i.e., a demand)
- compounds which degrade in water
In the planning of a slime control program, any chemical demand of process waters for the antimicrobial being used must also be considered. Failure to allow for the chemical demand may prevent attainment of the necessary threshold concentration and may lead to the failure of the treatment program. The compatibility of the antimicrobial with other treatments added to the water should also be considered.
Many system variables influence the behavior of microbes in the system, and the effects of antimicrobials can also be influenced by these variables. Therefore, careful consideration must be given to the determination of whether, when, and where to treat a cooling water system.
Cost is a primary criterion for selecting a slime control program. It cannot be determined without knowledge or estimation of the individual costs of chemicals, feed equipment, and labor required to apply and monitor the program, along with effluent treatment requirements. In addition, possible adverse effects of implementing the program must be weighed against those which would result if no treatment is made. Knowledge of component costs can help guide implementation of the program. For example, if labor costs are high, it may be more economical to feed an antimicrobial more frequently and reduce the amount of monitoring required. Each system must be considered individually, and seasonal adjustments may also be required.
CHARACTERISTICS OF NONOXIDIZING CHEMICALS USED IN COOLING SYSTEMS
Antimicrobials used for microbiological control can be broadly divided into two groups: oxidizing and nonoxidizing. Oxidizers, such as chlorine and bromine, are addressed in Chapter 27; nonoxidizers are discussed in the balance of this chapter.
Only a relative distinction can be made between oxidizing and nonoxidizing antimicrobials, because certain nonoxidizers have weak to mild oxidizing properties. The more significant difference between the two groups relates to mode of action. Nonoxidizing antimicrobials exert their effects on microorganisms by reaction with specific cell components or reaction pathways in the cell. Oxidizing antimicrobials are believed to kill by a more indiscriminate oxidation of chemical species on the surface or within the cell.
An understanding of the chemistries and modes of action of antimicrobials is needed to ensure their proper use and an appreciation of their limitations.
Two characteristic mechanisms typify many of the nonoxidizing chemicals applied to cooling systems for biofouling control. In one, microbes are inhibited or killed as a result of damage to the cell membrane. In the other, microbial death results from damage to the biochemical machinery involved in energy production or energy utilization.
Quaternary ammonium compounds (quats) are cationic surface-active molecules. They damage the cell membranes of bacteria, fungi, and algae. As a result, compounds that are normally prevented from entering the cell are able to penetrate this permeability barrier. Conversely, nutrients and essential intracellular components concentrated within the cell leak out. Growth is hindered, and the cell dies. At low concentrations, quats are biostatic because many organisms can survive in a damaged state for some time. However, at medium to high concentrations, quats can control the organisms.
Many antimicrobials interfere with energy metabolism. Because all microbial activity ultimately depends on the orderly transfer of energy, it can be anticipated that interference with the many energy-yielding or energy-trapping reactions will have serious consequences for the cell. Antimicrobials known to inhibit energy metabolism include the following:
- organotins
- bis(trichloromethyl) sulfone
- methylenebis(thiocyanate) (MBT)
- Beta-bromo-Beta-nitrostyrene (BNS)
- dodecylguanidine salts
- bromonitropropanediol (BNPD)
All of these compounds are effective when applied in sufficient concentrations. Dodecylguanidine salts also have surfactant properties, which probably contribute to their effectiveness.
The exact sites or reactions which are affected by such metabolic inhibitors are frequently unknown, although laboratory experiments may provide clues or indirect evidence for specific mechanisms. Tin and other heavy metals in sufficient concentrations cause proteins to lose their characteristic three-dimensional structures, which are required for normal function. Some antimicrobials, such as methylenebis(thiocyanate) (MBT) are believed to bind irreversibly to biomolecules, preventing the sequential reduction and oxidation these molecules must undergo in order to function.
Bromonitropropanediol (BNPD), a newer cooling water control agent, can be shown to catalyze the formation of disulfide bonds (R-S-S-R) between sulfhydryl groups (R-SH). Proteins contain sulfhydryls, and because enzymes are largely protein, it is possible to speculate that the formation of disulfide bonds between adjacent -SH groups may block enzyme activity. Many different enzymes contain sulfhydryl groups, so this antimicrobial may affect a wide range of microbial activities in addition to energy generation.
The mode of action of one common nonoxidizer cannot be categorized as either a surface-active or metabolic inhibitor. The active, dibromonitrilopropionamide (DBNPA), seems to behave somewhat like an oxidizing antimicrobial, reacting quite rapidly with bacterial cells. Studies of the interaction of radioactively labeled [14C]DBNPA with bacteria have shown that the 14C label never penetrates the cell, as would be necessary for it to become involved with energy metabolism. Instead, it binds strongly and rapidly to the cell walls of the bacteria. However, analogous studies with [14C]-Beta-bromo-Beta-nitrostyrene (BNS) have shown that the nitrostyrene penetrates the bacterial cell and accumulates within at concentrations far above the external concentration. Thus, although the mode of action of DBNPA is unknown, it most probably is unlike the other mechanisms known for nonoxiding antimicrobials.
Some antimicrobials used in cooling systems are compounds that spontaneously break down in water, thereby alleviating some potential environmental hazards. This chemical breakdown is often accompanied by a reduction in the toxicity of the compound. The compound can be added to the cooling water system, accomplish its task of killing the microbes in the system, and then break down into less noxious chemicals. Among the antimicrobials which have this attribute are BNS, MBT, DBNPA, and BNPD.
PROPRIETARY NONOXIDIZING CHEMICALS
The dynamics of microbial populations in cooling water systems are complex. In situations where one microbial group or species dominates, fouling problems can occur. In other instances, a balanced population mix can exist while no fouling is evident. One explanation for such observations is that when balanced populations coexist, they compete with each other for the available nutrients and control each other's growth. When one group successfully displaces the others, its growth can proceed without competition.
Because of such considerations, some proprietary antimicrobials are formulated to contain more than one active. Proper blending of actives can compensate for limitations in the spectrum of kill shown by one or more of the actives. For example, if antimicrobial A is effective for bacteria but poor for fungi, large amounts of A might have to be used to control potential fungal problems. However, if antimicrobial B is fair for bacteria and good for fungi, a combination of A and B would broaden the spectrum of control and thus be preferable to high concentrations of A alone.
With no increase in the amount of antimicrobial used, the power of a blend can exceed that expected from a simple additive effect. This greatly enhanced performance or synergism is obtained from only certain combinations of actives. Synergism allows microbial control at much lower combined concentrations of A and B than could be achieved with either A or B alone. Use of products composed of synergistic active blends can result in reduced treatment concentrations in the blowdown water, as well as cost savings.
The spectrum of control can also be broadened by sequential feeding of antimicrobials to a system: alternate feeding of two actives can have the same outcome as blending of the actives for simultaneous feeding.
Another variation to be considered is the possible proliferation of resistant microbes in the system. The resistant forms may arise spontaneously by mutation within the cooling system but are much more likely to originate outside of the system. The antimicrobial simply functions to reduce competition by nonresistant forms and permits the unchecked growth of the newly introduced resistant organisms. This is more likely to occur during treatment with a single antimicrobial active ingredient, because the probability of a microbe being resistant to more than one active is extremely low when the actives are dissimilar. Sequentially added and synergistically blended antimicrobials are probably equally effective in eradicating antimicrobial-resistant microbes from a cooling system.
Development of an effective antimicrobial program is aided by an understanding of the mode of action of the products, the system to be treated, and environmental effects. All of these factors play a role in the selection of an effective and economical microbiological control program that is safe for the environment.
Learn more about Veolia's microbiological control agents.
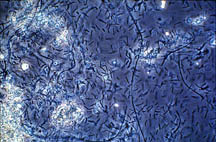
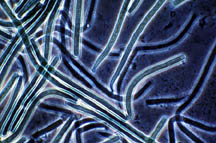
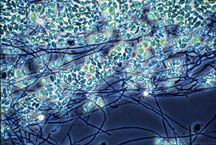
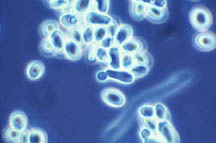
Figure 26-1. Some of the many microorganisms that colonize industrial cooling systems; (a) bacterial rods; (b) filamentous blue-green algae (now cyanobacteria); (c) unicellular green algae; (d) unicellular fungi (yeast); (e) filamentous fungi (molds).
|
(a)
|
(b)
|
|
(c) |
(d) |
|
(e) |
|
Figure 26-2. Extracellular polymers (visible in this scanning electron photomicrograph as a dehydrated fibrous network) may protect attached microorganisms.
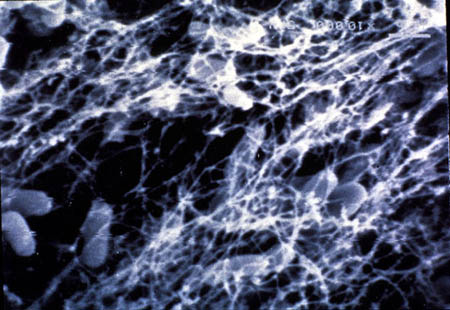
Figure 26-3. Microorganisms can contribute to corrosion of metals: (a) pitting attach visible in a cross section of stainless steel tube wall; (b) generalized surface attack associated with corrosive by-products of growing microbial colonies.

(a)

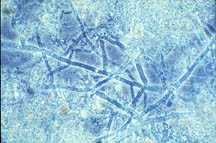
Figure 26-4. Microscopic examination of deposits can rapidly determine the principal microbial constituents and provide a photographic record.
Figure 26-5. Sulfate-reducing bacteria are detected by formation of black FeS precipitates in speciallly prepared liquid (a) or solid (b) media.

(a)

Figure 26-6. Cultivation of organisms sensitive to oxygen can be accomplished in this anaerobic glove box.

Figure 26-7. Microbial incorporation of radiolabeled nutrient, an indicator of microbial activity, is measured with this liquid scintillation counter.